Keywords
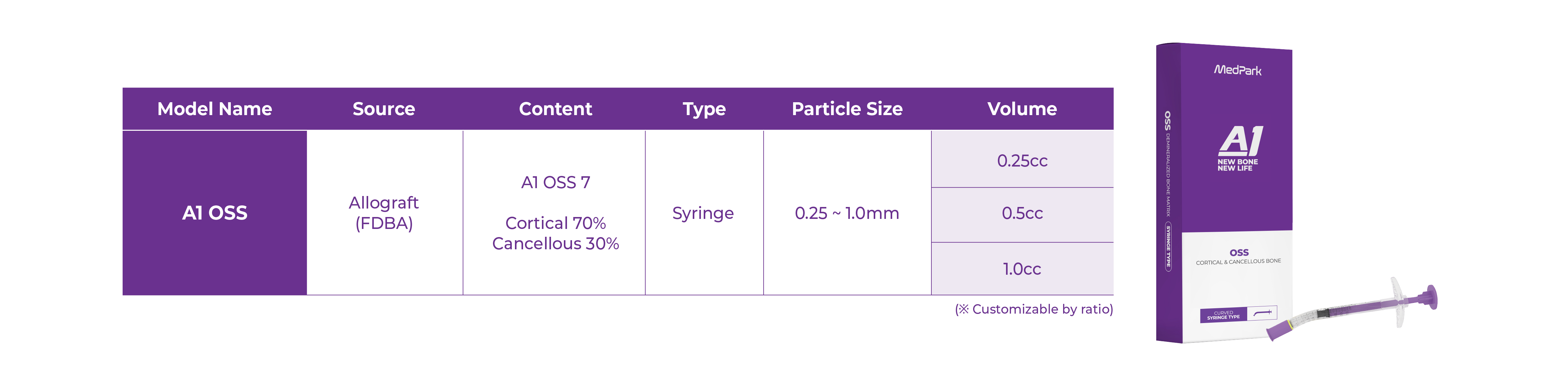
CORTICAL 70% + CANCELLOUS BONE 30%
-
Keywords
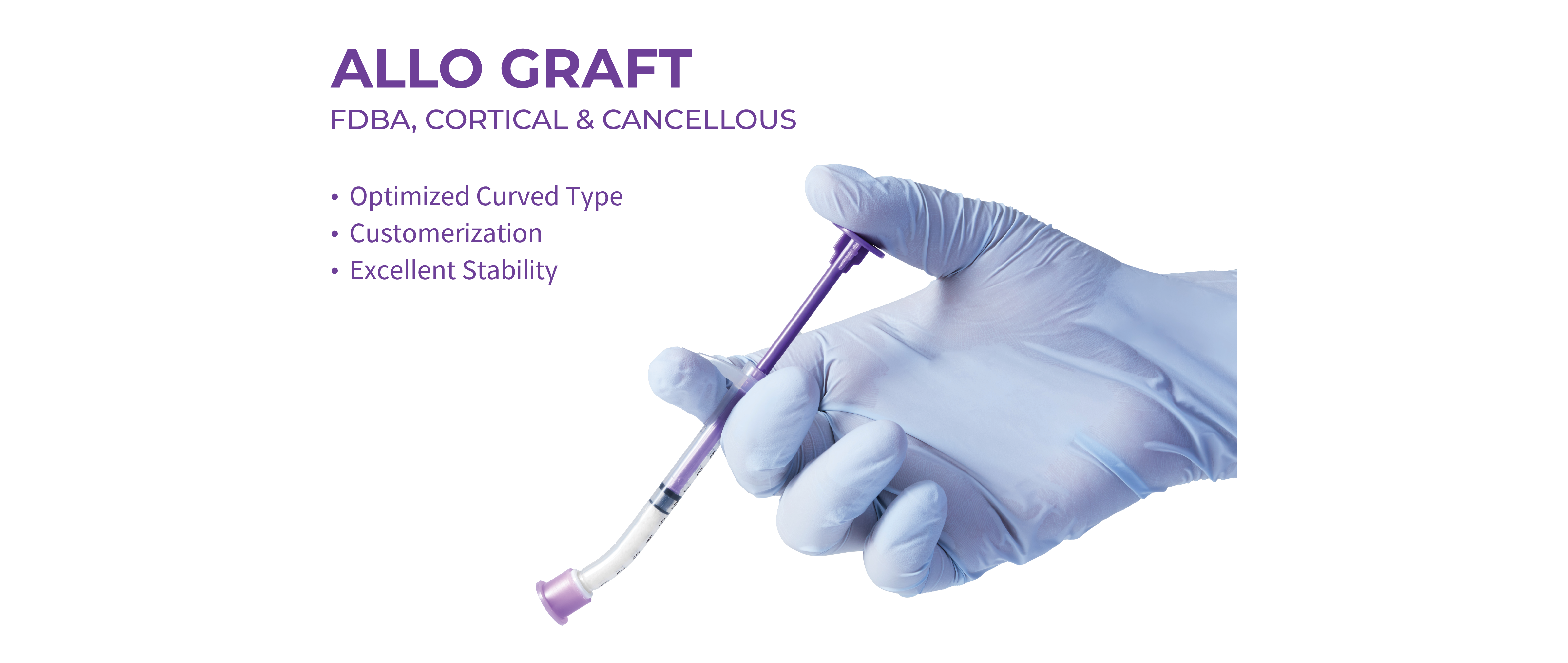
Donor Management According to The US GTPOwn ManufacturingOwn Production SystemPossession of Various types of ProductsExcellent New Bone Formation RateUser-Friendliness
Technical Data
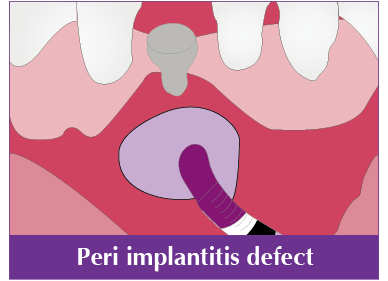
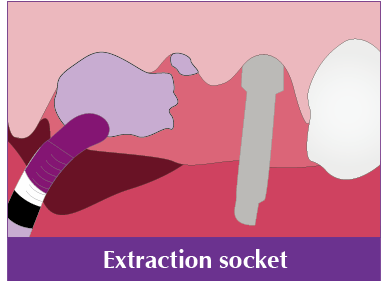
Sinus lift, GBR, Horizontal augmentation, Periodontal defect, Extraction socket, Peri implant defect
Sinus lift, GBR, Horizontal augmentation, Periodontal defect, Extraction socket, Peri implant defect
-
Intended Use
It is a biomaterial used for replacement, restoration, and reconstruction when bone is lost due to trauma or osteoporosis.
-
Preoperative Preparation
1. Do not transplant at the site where is infected or an infection is in progress.
2. According to the Human Tissue Act, the tissue transplant result record must be delivered within one month after tissue transplantation.
3. The tissue should be used immediately after rehydration if possible.
4. Disposable, do not reuse.
5. Check the sterilized packaging before use.
6. Do not use a product that expiration date has passed.
7. If contaminated products are found, discard or return them.
8. It should be used bedoctors and dermatologists, and use other than the intended use is prohibited. -
Directions for Use
This product must be stored at room temperature (1~30℃, 34~86℉) and individually hydrated.
1. Vial Type
1) Open the outer packaging (Box packaging)
2) Remove the Tyvek packaging attached to the blister and take out the vial
3) Check if the vial is damaged and remove the double cap
4) Rehydrate the tissue in a container with a sterile solution stored at room temperature
5) The tissue should be used as soon as possible after hydration.
2. Syringe Type
1) Open the outer packaging (box packaging) and take out the blister
2) Remove the Tyvek pouch (secondary packaging) attached to the blister and take out the inner blister
3) Remove the Tyvek pouch (primary packaging) attached to the blister and take out the syringe
4) Remove the outer protective cap (rubber cap) so that the inner stopper (mesh cap) inside the syringe is not removed ⑤ Discard the used syringe together with the contents -
Storage and Expiration date
This product can be stored for up to 5 years in an unopened package at a dry room temp(1~30℃, 34~86℉)avoiding direct sunlight.
-
Precautions
This product is produced from donated human tissue and should be used only once per patient.
Manufacturer
Tissue bank, MedPark| #904, #913, #916, #1007, #1008, #1009, #1010, 13-gil 25, Seonyuro, Yeongdeungpo-gu, Seoul, Republic of Korea.|TEL : 82 2 2257 8330
Sepcifications